usp class vi compliant
USP Class VI materials EPDM Silicone Fluorocarbon and Perfluoroelastomer 24 materials which are compliant to FDA 21 CF R1772600. In addition SIMONA PP-H USP Class VI sheet delivers high chemical and corrosion resistance excellent surface.
USP Class VI materials are available in 70 durometer EPDM Silicone and Viton.
. Recent politicization of the COVID-19 vaccine development and approval process has led to a concerning loss of confidence in vital governmental institutions designed to protect the public from harm. Master Bond systems are very versatile and can be used for both disposable and reusable medical devices. SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931.
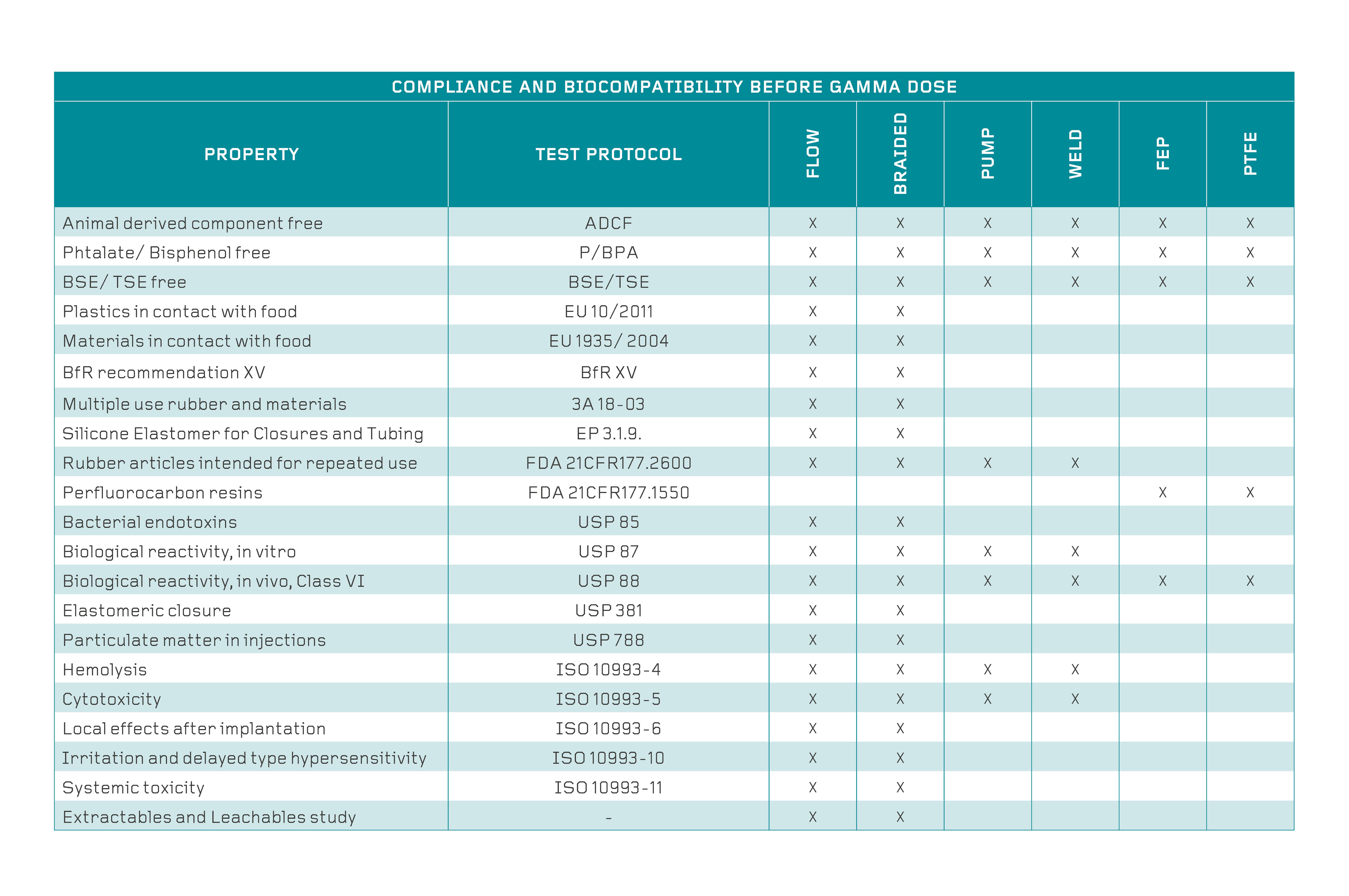
So here is a new one - a customer has requested us to conduct testing compliant to USP Class VI and ISO10993-1 compliant. Sterile and diaphragm valves have USP Class VI PTFE material in them and sanitary pumps require Class VI O-Rings and sealing material. Many plastics manufacturers find it advantageous to have their materials classified especially if their plastic resins are a likely candidate to be used in medical devices.
Manufactures standard and custom size o-rings compliant to the US. SIMONA PP-H USP Class VI sheet material is easy to clean and disinfect using most hospital grade cleaners and disinfectants. The USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet tighter requirements for leachates.
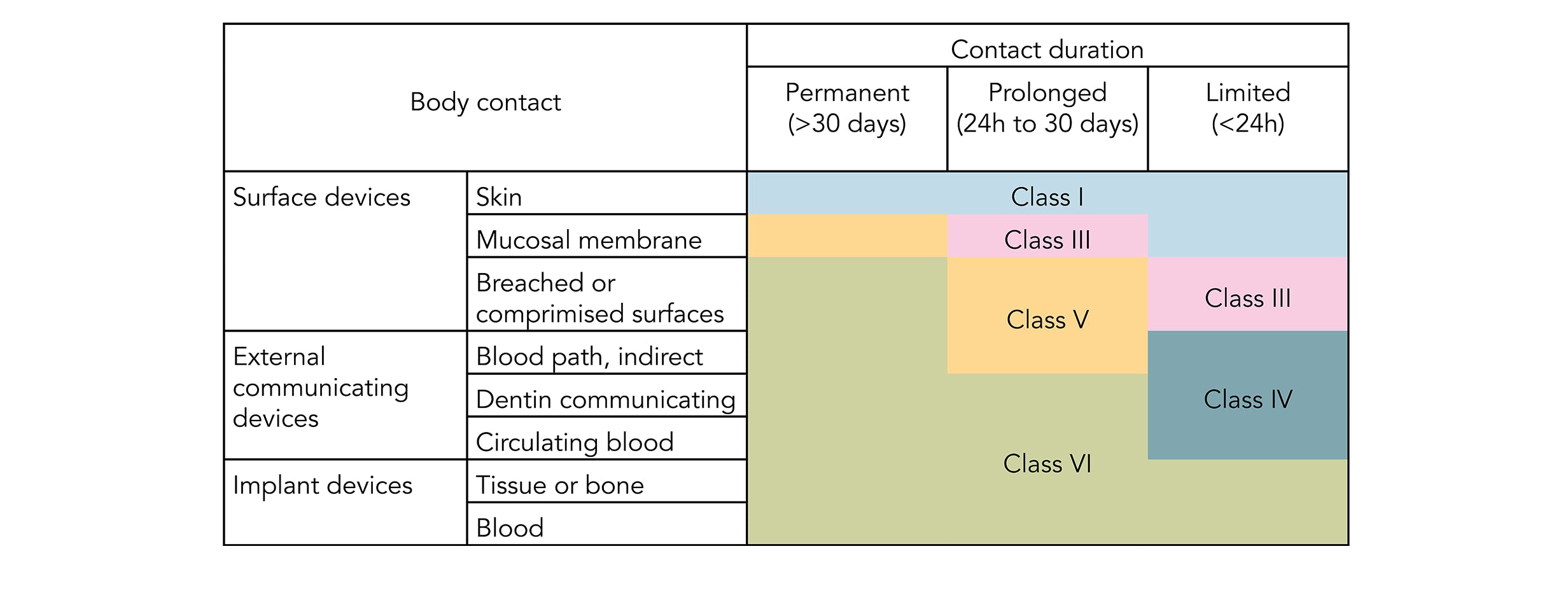
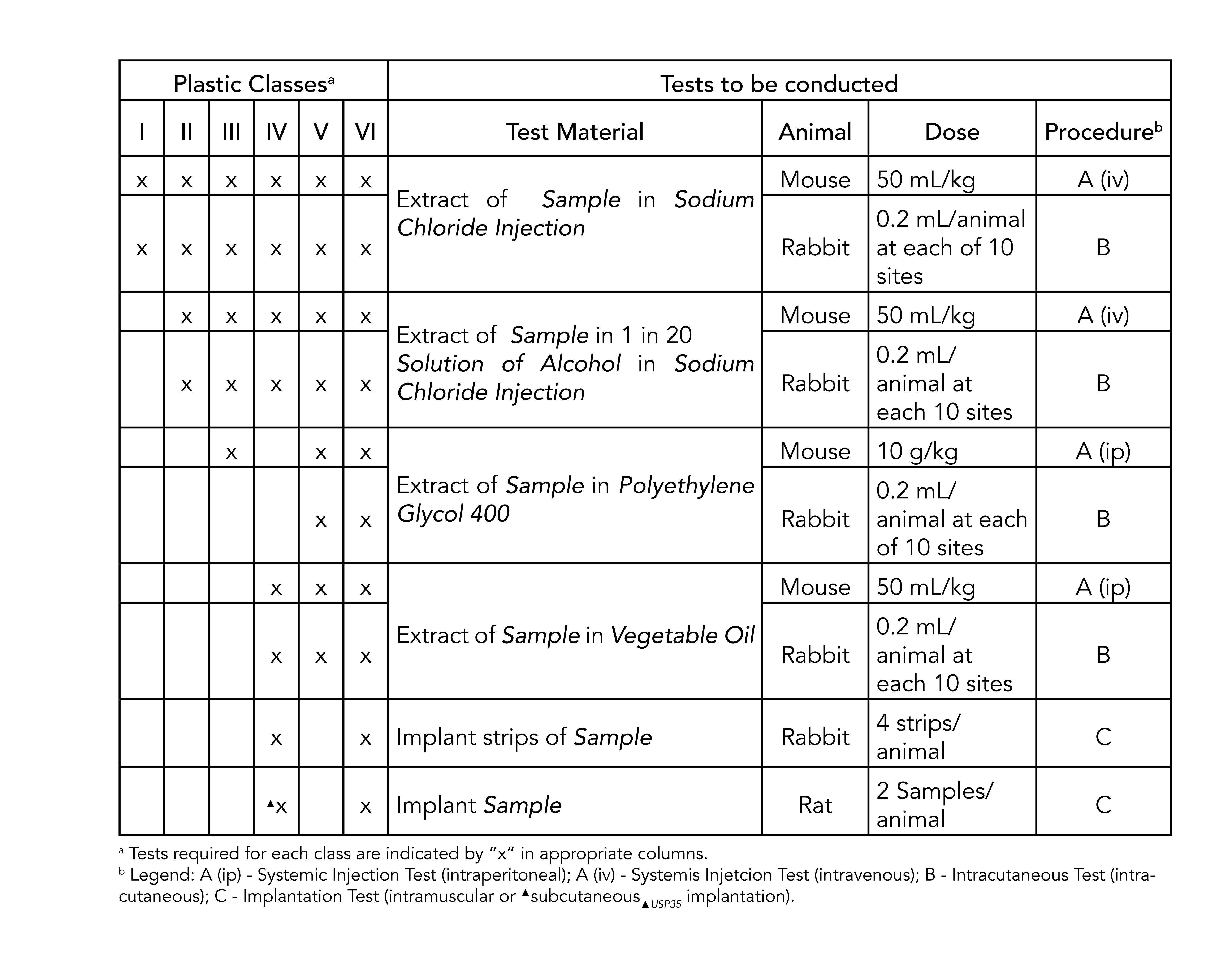
USP Class VI refers to a set of biocompatibility testing requirements from the US. Specifically USP publishes test instructions for the plastics polymers and elastomers that are used in medical devices and surgical equipment. The USP defines six plastics classes from class I to class VI with class VI being the most rigorous and most frequently requested certification.
Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal. United States Pharmacopeia USP 26 NF21 2003 Class VI Three chapters are applicable to elastomers plastics and polymeric materials. Sil 714001 USP class VI Silicone 1 70 Yes transl.
Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials. Specially formulated for long term sealing. Compliance with the USPNF.
USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet tight requirements for leachates. I know that performing a USP Class VI test even for a 30 day period will still not perform to ISO10993-1 per General ProgramBluebook Memo G95-1 We are. What does compliance with USPNF standards mean.
Compliance to USP Class VI is often requested by users in the biopharmaceutical and medical industries. Food and Drug Administration FDA. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials.
7 USP Class VI materials EPDM silicone fluorocarbon and perfluoroelastomer 24 materials which are compliant to FDA 21 CFR1772600. But Dursan is one of the first CVD silicon coatings to achieve USP Class VI compliance. Class Compliance to USP Class VI is often requested by end users.
All these special grade products have passed this rigorous test. Testing for compliance involves an assessment of the effects of the material and extractables on tissue. USP Class VI compliant compounds are formulated with ingredients providing certifiable biocompatibility and low extractables for system toxicity and purity.
Many plastics manufacturers find that it is beneficial to be USP Class VI certified especially if the end use is a medical device. Sil 714002 USP class VI Silicone 1 70 Yes transl. ADI-free certifies that the raw materials used in production of the elastomer contain no Animal Derived Ingredients ADI.
Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds. E6A53 A representative article comprised of two halves of E553 and a splice utilizing E6A53 has been one time tested for USP Class VI compliance This article was manufactured using procedures typically required to produce final parts. What is ADI-Free BSE-Free TSE-Free.
IEGeek - 2006. There are six classes VI being the most rigorous. Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements.
USP Class Testing standards are determined by the United States. According to USP United States Pharmacopeia there are six plastics classes ranging from I to VI with class VI being the strictest. USP Class VI and FDA White List Silicone and Organic Elastomer Compounds for.
Theres even a silicon carbide surface thats compliant and various release agents used in the manufacture of silicone plastics are compliant. Testing for compliance involves an assessment of the effects of the material and extractables on tissue. When production of the elastomer contain no ADI with respect to source manufacture and treatment they cannot.
Standards are published in the US Pharmocopeia and the National Formulary USP NF. Compounds made without animal-derived ingredients BSETSE concerns. United States Pharmacopeia USP 26 NF21 2003 Class VI.
An article of commerce that is recognized in the USPNF complies with USPNF standards when it meets all of the requirements stated in the articles monograph applicable General Chapters and the General Notices with monograph requirements. Specially formulated for long term sealing. USP Class VI Certificate of Compliance Silicone Compound.
Pharmacopoeia USP Class VI requirements. There are plenty of silicone products and other medical grade plastics that are USP Class VI compliant. AFT Fluorotec can manufacture a wide range of components using our USP Class VI PTFE compliant material and are supplying customers worldwide to meet their requirements.
Eastern Seals UK Ltd can provide a variety of USP Class VI compliant O-rings sanitary gaskets and seals in various elastomer types including EPDM SILICONE FKM VITON and PTFE TEFLON. Pharmacopeia USP a non-profit organization whose standards inform decision-making at the US. Typical applications for our FDA NSF 51 USDA materials are disposable medical.
Table 1 shows our standard programme FDA compliant com-pounds which can be produced in a few days. Compliance to USP Class VI is often requested by end users.

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Usp Class Vi Foster Corporation

0 75 3 4 Id Fda Usp Class Vi Platinum Silicone W Polyester Braid Food And Pharma Grade Flex Technologies Incorporated

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc

What Is Usp Class Vi Testing Tbl Plastics
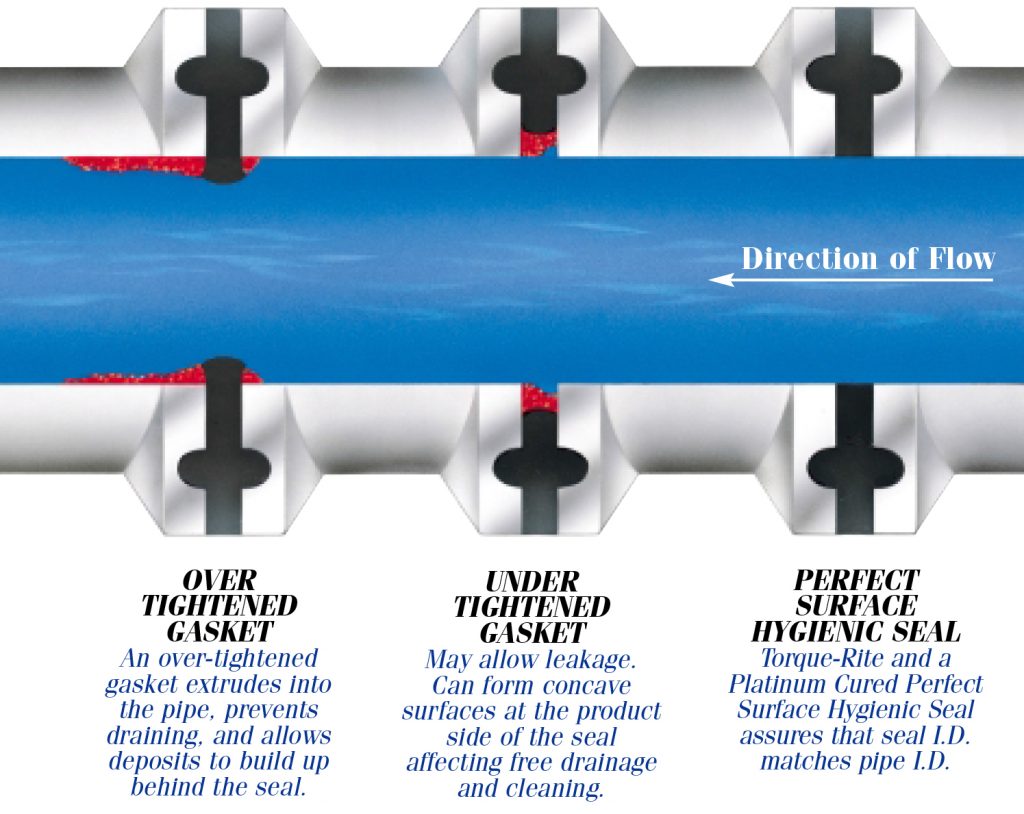
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Dursan Passes Usp Class Vi Testing Why Is That Important

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Usp Class Vi O Rings And Seals Eastern Seals Uk Ltd

Cpc Usp Class Vi Compliant Polysulfone High Flow Quick Disconnect Couplings Cole Parmer